Evaluation of vaporization enthalpy helpful in studying liquid-gas transitions
A paper by Kazan Federal University was published in early access in Journal of Molecular Liquids.
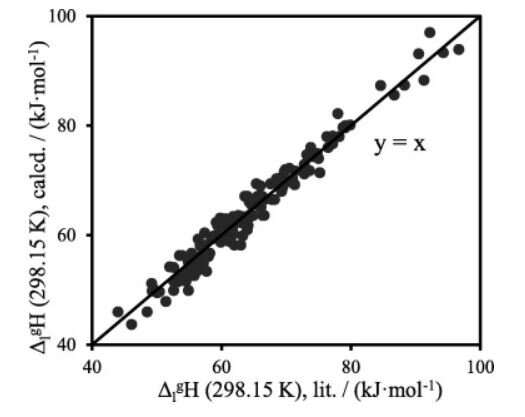
The work was aimed at the prediction of the vaporization enthalpies of alkylaromatic compounds and their derivatives. This is a development of the recently proposed system of methods for evaluation of the phase transition enthalpies of aromatic and heteroaromatic compounds, on the one hand, and aliphatic compounds, on the other. According to the proposed approach, the vaporization enthalpy of an aliphatic compound carrying aromatic substituents can be derived from the vaporization enthalpy of an aliphatic compound, serving as a backbone, and a contribution of an aromatic fragment, which is also derived from the vaporization enthalpy of a pure aromatic compound. The additivity of the vaporization enthalpies of the fragments may fail in two cases.
First, non-additivity is possible when polarizability due to π-conjugation is changed due to combination of the aliphatic and aromatic fragments. Another source of non-additivity is change in intermolecular hydrogen bonding strength. Independent procedures for accounting for both factors were proposed. Reliability of the proposed approach was justified by comparison with a large literature dataset on the vaporization enthalpies of alkylaromatic compounds. Its accuracy was competitive with the accuracy of experimental techniques.
Vaporization enthalpy is one of the key thermodynamic parameters of liquid-gas transition. This quantity is widely used in chemical industry when calculating heat balances. It also defines the temperature regime of distillation processes. The developed approach can be readily used by the specialists in the industry for evaluation of the properties of poorly studied compounds and requires only the data on the molecular structure.
The present approach enables calculating the vaporization enthalpies of organic non-electrolytes at the reference temperature commonly used in thermodynamics, namely 298.15 K. The KFU team developing it in order to apply to the calculation of the thermodynamic parameters at arbitrary temperatures. The developments are based on the critical analysis of the literature data, new measurements, and theoretical advances.
More information:
Additivity of vaporization enthalpy: Group and molecular contributions exemplified by alkylaromatic compounds and their derivatives
www.sciencedirect.com/science/ … ii/S0167732221021966
Provided by Kazan Federal University